Atomic orbitals are the regions in space where it is more probable to find an electron. This is somehow synonymous to the definition of molecular orbitals though for the latter, the electrons belong to the entire molecule and not to its individual atoms. Another way to put it is that an electron in an atomic orbital is under the influence of only one nucleus of the atom while an electron in a molecular orbital is under the influence of two or more nuclei, depending upon the number of atoms present in the molecule.
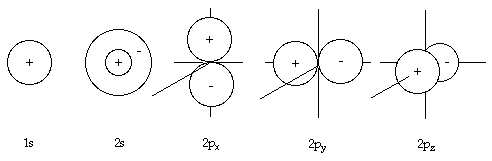
The 1s atomic orbital has no node (region where there is zero probability of finding an electron) and has the lowest energy. The 2s atomic orbital is bigger than 1s and is with one node, thus it has higher energy compared to 1s (as more nodes mean higher energy for the orbital). On the three 2p orbitals, the x, y, or z label indicates along which axis the two lobes are directed. It is also understood that in orbital labels, the number tells the principal energy level while the letter tells the shape. The letter s means a spherical orbital; the letter p means a two-lobed orbital.
All the topics above have already been discussed during our Chem14 days thus it wasn't that hard to understand that part of the lecture. The more complicated orbitals though need a bit more refreshing as they are much harder to visualize. If you want to get a better glimpse of them, click here. :)

0 comments:
Post a Comment