Conformations are 3-dimensional shapes that can be taken by a molecule by rotating about single bonds.
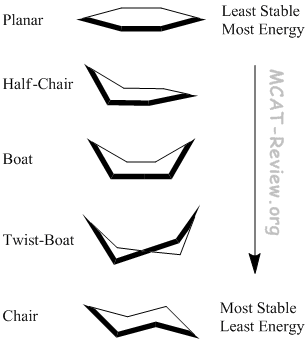
- In the planar conformation, everything is eclipsed. In an eclipsed conformation, the bonds have dihedral angles of zero degrees. This maximizes the energy and leads to instability. Steric hindrance of eclipsing interactions also lead to torsional strain or the resistance to rotation about a single bond.
- In the chair conformation, everything is staggered. In a staggered conformation, the bonds have dihedral angles of 60 degrees. This minimizes the energy and thus leads to more stability.
- All the conformations in between are partially eclipsed.
- The Boat conformation has Flagpole interactions because axial groups attached to the head and tail of the boat clash.
- The Twist-boat conformation lessens these Flagpole interactions in addition to reducing the number of eclipsed interactions.


0 comments:
Post a Comment